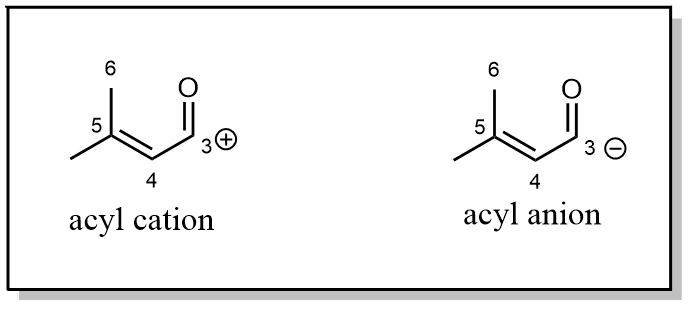
The carbonyl group, is a perfect electrophile, often, it is recognized as a partially positively charged group. But from some retrosynthetic plan, people found they already had an electrophile thus requiring the acyl group to act like an anion that needs to be partially negatively charged.
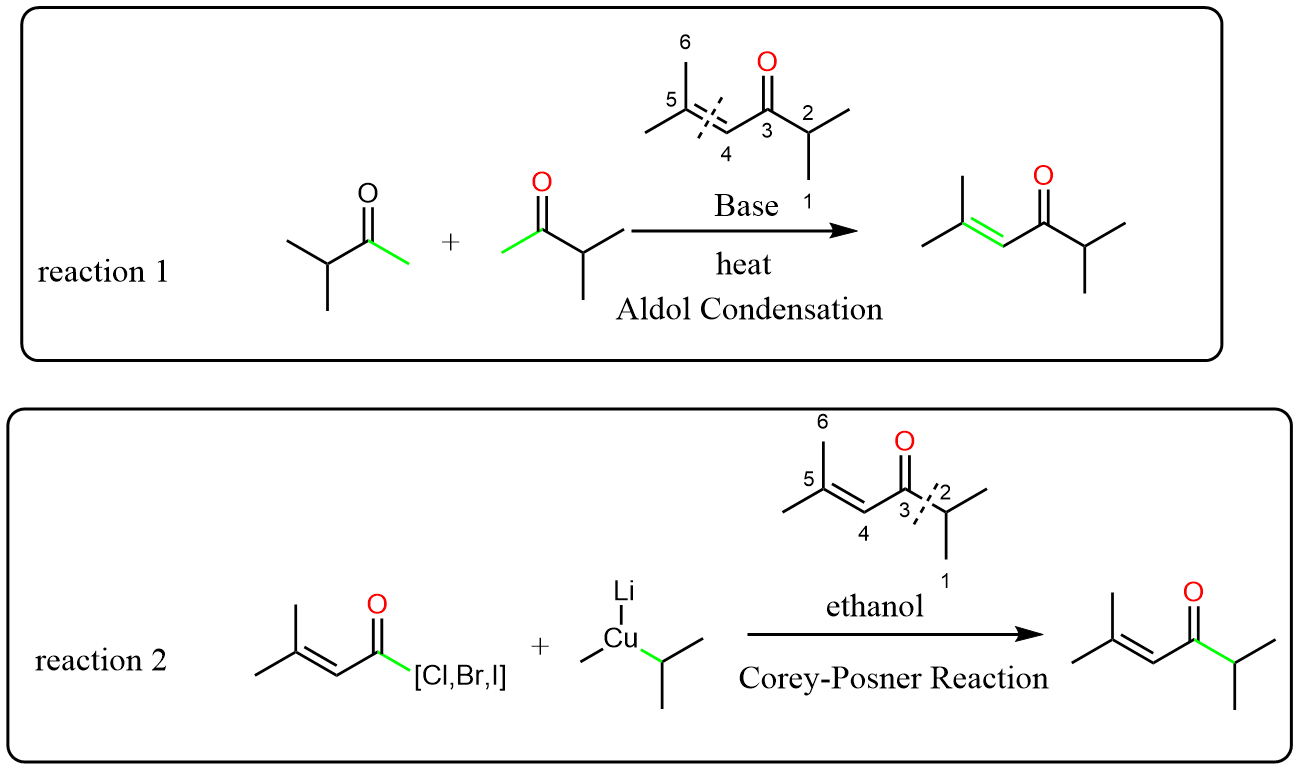
Now let have a look at a simple retrosynthesis of a compound. There are many potential reactions we can apply here to obtain this compound. The simplest one maybe is the aldol condensation, if we break down between carbon 4 and 5.
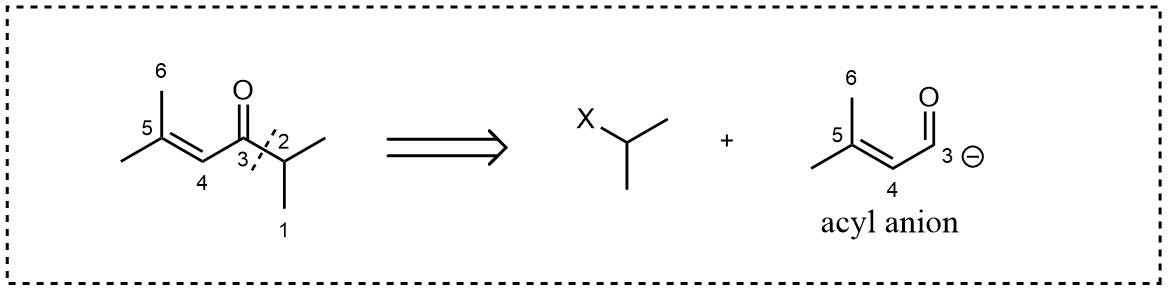
If we decide to break down carbon 3 and 2, things begin to be a bit complex, if you want to use metal, a reaction like Corey-Posner Reaction might be applied. But what if you don’t want to use metal?
We need a potential reaction 3 as shown below.One can assume that the right-hand side subunit being an alkyl halogen, as an electrophile, while the left-hand side an acyl anion. The question is how could we obtain such a species in practice, given the acyl group’s positively charged nature.
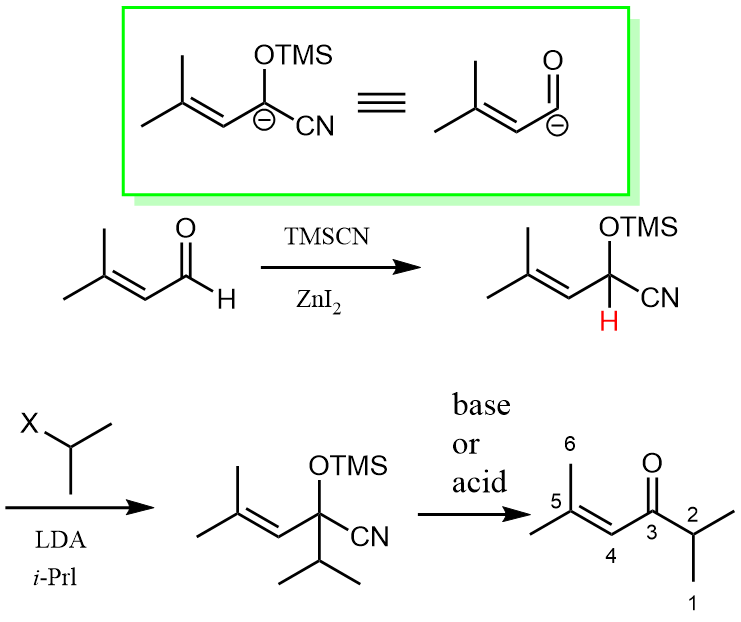
One way to do this is taking TMSCN as a masking group by addition to the acyl group, which can then be deprotonated, serving as a negatively charged nucleophile instead of the electrophile. After the nucleophilic addition to alky halogen, it is then unmasked back to an acyl group.
As the above green box shows, we take the TMSCN masked anion as an acyl anion equivalent in practice. The reason why this could work nicely is partly due to the strong electron-withdrawing nature of the CN group, which can stabilize the negative charge after LDA deprotonation.
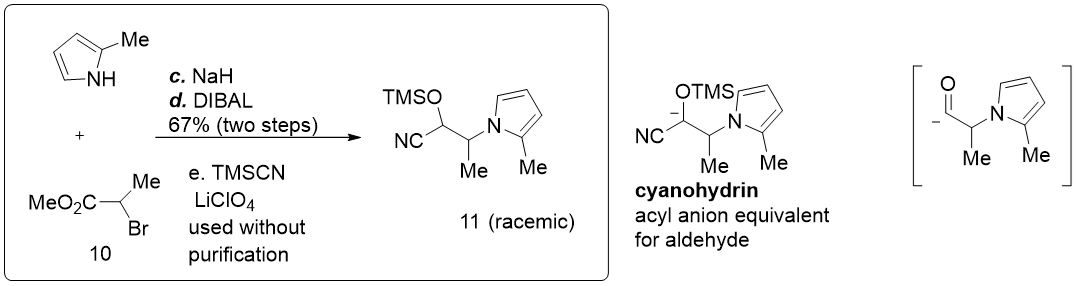
This strategy was applied in the total synthesis of (-)-Curvulamine by Maimone Group at UC, Berkeley. We only show part of their synthesis plan involves our topic here.
What they need is an acyl group similar to what we have discussed above, they also adopted the same strategy, using TMSCN as a masking group, in a three-step one-pot reaction, first an SN2 alkylation to 2-methyl pyrrole N atom, then a reduction from ester to aldehyde, followed by the TMSCN masking.
For the whole process of Curvulamine synthesis, you can read the total synthesis of (-)-Curvulamine.